D2R - Design to Regulatory for MedTech
End-to-End Regulatory & Compliance Consulting for Medical Devices, Diagnostics and Digital Health
Founded in 2018 (formerly Elite QARA Consulting), D2R is a specialized advisory firm delivering end-to-end Healthcare and MedTech services with a strong focus on global market access. Our multidisciplinary team of 30+ experts brings deep domain knowledge across Medical Devices, In Vitro Diagnostics (IVDs), and Digital Health, enabling efficient and compliant product development for medical technology manufacturers.
We offer customized, regulatory-aligned solutions across the product lifecycle, including new product development, quality management system (QMS) consulting, clinical trial planning and execution, regulatory strategy and submissions, facility setup and compliance, digital health integration, information security, and India market entry strategy.
Through our integrated approach and cross-functional expertise, we accelerate time-to-market while ensuring regulatory robustness and operational scalability.
Over the past seven years, we have successfully engaged with more than 100 clients and executed over 250 projects globally, spanning startups, SMEs, and multinational corporations.
Our Services
At D2R, we offer a comprehensive suite of services to support medical device innovators throughout the entire product lifecycle — from initial concept and feasibility studies to regulatory approval, manufacturing, and commercialization. Our multidisciplinary team brings deep expertise in biomedical engineering, clinical validation, quality systems, and global regulatory strategy (including FDA, MDR, and ISO 13485). We work closely with clients to de-risk development, navigate complex compliance requirements, and accelerate time-to-market — enabling the successful delivery of life-changing medical technologies to patients with confidence.
From concept to creation, we offer tailored solutions for designing and prototyping innovative medical devices. Leverage our expertise in engineering, material science, and compliance to fast-track your product development journey.
Our services span functional testing, biocompatibility, and risk assessments. including comprehensive testing solutions for medical devices to ensure safety, performance, and regulatory compliance.
Ensure flawless execution of quality processes and compliance with global standards through our expert guidance.
We can help you streamline clinical study designs, execution, and data analysis with our in-depth expertise in medical device trials.
Navigate complex regulatory landscapes with confidence. We assist with certifications, submissions, and compliance across markets.
Transform your vision into reality. We help design, build, and validate state-of-the-art medical device manufacturing facilities.
Leverage our expertise in developing cutting-edge healthcare apps tailored to patient care, data analytics, and medical workflows. From wireframing to deployment, we cover every aspect.
Transform your vision into reality. We help design, build, and validate state-of-the-art medical device manufacturing facilities.
We can get you access to India’s burgeoning medical device market with strategic support for product registration, compliance, and distribution.
Stay ahead of legal and regulatory requirements with our comprehensive statutory compliance services for medical devices and healthcare technologies.
D2R Global Consulting – Focus Sectors & Expertise
At D2R Global Consulting, we empower healthcare and life science innovators across a spectrum of high-impact domains. Our cross-functional expertise, spanning regulatory, clinical, quality, and engineering, enables us to guide startups and enterprises from idea to commercialization with confidence and compliance.
Our Focus Area
Our Focus Area
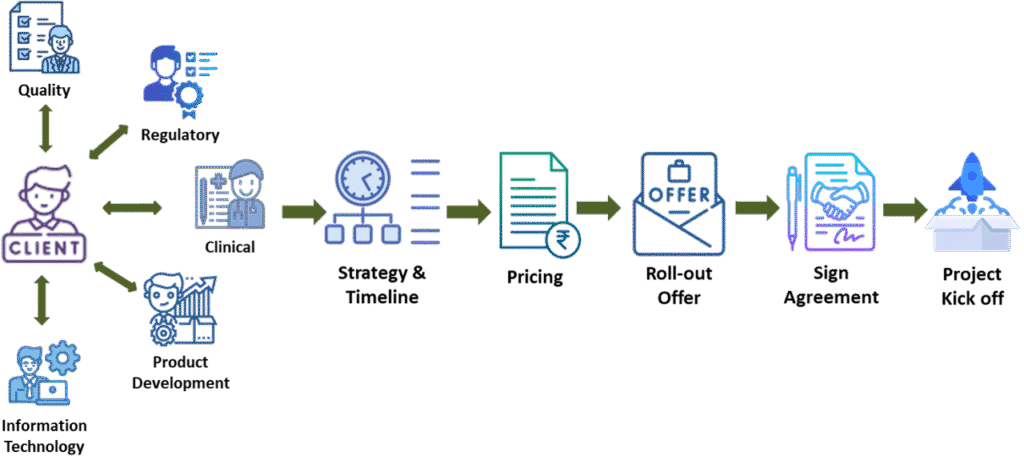
How We Operate?
D2RGC follows a streamlined process to turn your ideas into reality. We begin by understanding your requirements through detailed consultations. Our team then conducts thorough research and planning, creating comprehensive proposals. Once the proposal is approved, we formalize the agreement and move into the development phase. Throughout this journey, we ensure clear communication and collaboration, leading to the successful delivery of your project. Trust us to transform your vision into a tangible and innovative solution.
Our Journey
Our Team

Mr. Rahul Dutta
Co-Founder & Head-SW Engg

Mr. Chiranjeevi N
Head — Medical Device & Diagnostics

Dr. Baraneedharan Ulaganathan
Head — Biotech/ Biopharma

Dr. Alphy Zachson
Head —Clinical Affairs
Advisors
Testimonials
Device Prototyping
Partnering with D2R Global Consulting on the HealthCube project was an incredible experience. Their expertise in medical device prototyping and regulatory compliance took our idea from concept to a fully functional prototype in a seamless and timely manner. They paid meticulous attention to detail, especially during the material sourcing and design iterations, and their collaborative approach made the entire process smooth. Thanks to D2R, we are now confidently moving forward with clinical validation, knowing we have a solid foundation for a successful market launch.IVD Kit Clinical Study
Our Semen Detection Kit’s successful clinical validation was made possible in large part by D2R Global Consulting. Their team’s meticulousness and proficiency in trial execution enabled us to successfully negotiate intricate regulatory environments and produce reliable, accurate outcomes. Their end-to-end help from study design to regulatory submissions gave us peace of mind about our product’s performance and safety. Their services have greatly satisfied us.Manufacturing Facility Development
When building our ISO 6, 7, and 8 cleanroom facilities, D2R Global Consulting’s knowledge of cleanroom design and regulatory compliance was crucial. From conceptual design to final validation, their team delivered a flawless solution, guaranteeing that our facility complied with the strictest requirements needed to manufacture medical devices. They demonstrated remarkable attention to detail in air quality control, space optimization, and regulatory compliance. We have a fully functional, ISO-certified cleanroom now, thanks to D2R, which has allowed us to expand our production while still adhering to international standards.SaMD, Class C
We received professional assistance from D2R Global Consulting at every turn during the CDSCO approval procedure for our SaMD diagnostic software. The D2R team offered crucial assistance from the documentations for application submission to assisting with the inspection procedure, guaranteeing that all regulatory criteria were fulfilled. We were able to easily manage the complexity thanks to their team’s practical approach and thorough comprehension of CDSCO requirements. Because of their hard work, we were able to receive approval and confidently position our software for the Indian market.India Market Entry Assistance
In order to handle the intricate regulatory environment in India for our surgical dressing solutions, we partnered with D2R Global Consulting. Their knowledgeable assistance with market research, strategy execution, and CDSCO registration was priceless. We confidently launched our product in India because of their team’s extensive market expertise and their abilities to navigate the logistical and regulatory challenges. Our market entry was quick and easy thanks in large part to the D2R team.510(k) Clearance
Working with D2R Global Consulting for our 510(k) submission was a game-changer. Their team guided us through every step of the FDA process, from preparing detailed documentation to responding to FDA queries. They helped us identify predicate devices, ensured our clinical data met regulatory standards, and addressed every aspect of compliance with precision. Thanks to their support, we are in the process of applying 510(k) clearance for our ‘Epilepsy detection software’ and are now confident in entering the U.S. market.Combination Medical Device
D2R Global Consulting played a pivotal role in helping us in understanding the combinational device regulations in EU, USA and India. Their expert guidance aligned our Quality Management System with our client needs. Thanks to their dedicated team, we are now equipped to confidently expand our reach to global markets, adhering to the highest standards of product safety and performance.MD-15 Import License
D2R Global Consulting’s expertise was crucial in helping us secure the CDSCO import license for our medical device. Their structured approach to strategy, advisory, and team coordination ensured a smooth and hassle-free process. With their support, we have secured an import licence for one of our clients.Manufacturing License, Class B
D2R Global Consulting provided exceptional support in helping us secure the CDSCO manufacturing license for our AI-enabled comprehensive wound infection assesment and monitoring device. Their expertise in regulatory compliance, site preparation, and coordination with CDSCO officials ensured a seamless process from start to finish. With their guidance, we achieved approval efficiently and are now ready to manufacture and deliver our device to meet market needs. We highly recommend D2R for their professionalism and expertise.Device Testing
D2R Global Consulting provided end-to-end support in testing our endo-tracheal tube, ensuring compliance with ISO and IEC standards. Their expertise in electrical, biological, and mechanical testing protocols, coupled with detailed reporting and actionable feedback, made the process seamless. Thanks to their efforts, we are now ready to launch a globally compliant device.Refurbished devices regulation
D2R Global Consulting’s expertise was invaluable in setting up our refurbished medical device process. Their guidance on regulatory compliance, facility setup, and documentation made the entire process seamless. Thanks to their support, we are now confidently importing and refurbishing devices while meeting all Indian regulatory requirements.MD-42 wholesale license
D2R Global Consulting made the process of obtaining our MD-42 wholesale license seamless and efficient. Their expertise in navigating regulatory requirements, preparing the necessary documentation, and liaising with the licensing authority ensured a smooth approval process. Their proactive guidance and professionalism have been invaluable, and we highly recommend their services for anyone seeking regulatory support.ISO 13485 certification
Throughout our company’s ISO 13485 certification procedure, D2R Global Consulting offered outstanding advice. Their knowledge of quality management systems, careful documentation preparation, and useful suggestions made sure that we were in compliance without any problems. We were able to successfully align with international medical device standards thanks to the professionalism and practical assistance of their team.NABL accreditation (ISO 15189)
We received knowledgeable assistance from D2R Global Consulting during our diagnostic laboratory’s NABL accreditation (ISO 15189) procedure. Their in-depth knowledge of quality management systems, practical assistance with documentation, and unambiguous direction on adhering to managerial and technical specifications were priceless. We successfully obtained our national accreditation with their help, and we are now known for upholding the highest standards in medical testing.ISO 9001 certification
D2R Global Consulting provided us with exceptional guidance and support throughout the ISO 9001 certification process. Their expertise in quality management systems and meticulous approach to documentation and compliance made the entire journey seamless. The team’s practical recommendations and hands-on assistance ensured that we met all requirements efficiently. We highly recommend D2R for their professionalism and in-depth knowledge of certification processes.GMP compliance
Our mammalian cell culture laboratory’s GMP compliance was made possible in large part by D2R Global Consulting. Their knowledge made sure that we easily complied with regulatory requirements, from establishing the lab infrastructure to aligning our SOPs with GMP standards. Achieving compliance was made possible by their careful attention to paperwork, facility design, and quality management systems. We strongly advise anyone wishing to create and manage GMP-certified facilities to use their services.AI/ML based software development
For the development of our AI/ML-based cancer pathology imaging software, D2R Global Consulting offered thorough regulatory guidance. Their knowledge made sure our product complied with the most recent regulatory frameworks, including risk management, clinical validation, and standards like ISO 13485 and IEC 62304. Their tailored recommendations streamlined the development process, helping us build a robust, compliant solution ready for market entry.MDR CE certification (MDD to MDR remediation)
D2R Global Consulting’s expertise was instrumental in helping us transition from MDD to MDR CE certification for our medical device. Their thorough understanding of the new EU Medical Device Regulation (MDR), gap analysis, and remediation strategies ensured a smooth and efficient process. They provided tailored guidance on updating technical documentation, conducting clinical evaluations, and aligning with post-market surveillance requirements. Thanks to their support, we successfully achieved MDR compliance, securing our product’s continued market presence in the EU.IVDR CE certification (IVDD to IVDR remediation)
Our in vitro diagnostic instruments’ move from IVDD to IVDR CE certification was made possible with outstanding assistance from D2R Global Consulting. A smooth remediation process was guaranteed by their thorough understanding of the new IVDR criteria, which include improved clinical evidence, performance reviews, and post-market surveillance. They updated our technical documentation, carried out a comprehensive gap analysis, and helped us comply with the strict IVDR criteria. Their knowledge helped us guarantee our product’s place in the EU market and successfully obtain the CE certificate.IND (investigational new drug) Regulatory Strategy
D2R Global Consulting provided expert guidance in crafting a comprehensive IND (Investigational New Drug) regulatory strategy for our drug development program. Their team’s in-depth understanding of regulatory pathways, clinical trial design, and compliance requirements enabled us to create a roadmap tailored to our product’s needs. They guided us through compiling the IND dossier, including protocol design, safety assessments, and CMC (Chemistry, Manufacturing, and Controls) documentation and offered valuable insights on preclinical data requirements, risk mitigation strategies, and the regulatory expectations for future submission and trials. Thanks to their strategic approach, we are now well-prepared to advance our drug development with confidence.Our Happy Customers